Possible Treatment for Damage From COVID-19 and Long COVID Using a Monoclonal Antibody Called 5B8
The nature paper “Fibrin drives thromboinflammation and neuropathology in COVID-19” discusses the role of fibrin in COVID-19 and its complications, particularly how it contributes to inflammation and damage in the brain and lungs. It also highlights a possible treatment to prevent and stop the damage.
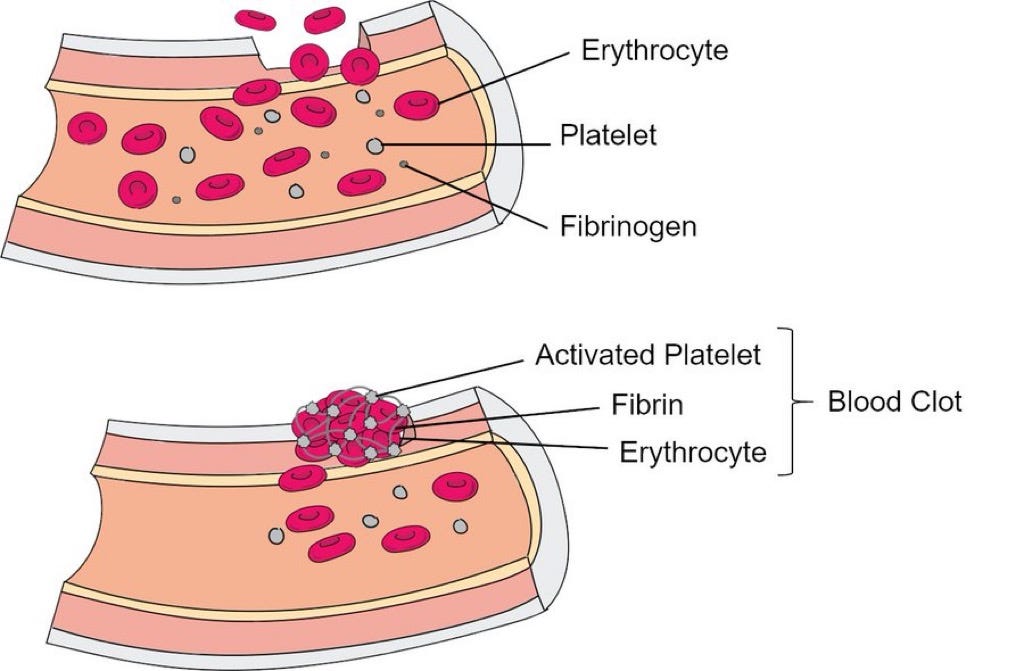
1. Fibrin and COVID-19: Fibrin is a protein involved in blood clotting. In COVID-19, fibrin plays a key role in forming clots that not only affect blood flow but also drive inflammation and damage in the body. This can lead to serious complications, like blood clots in the lungs and brain, which are seen both during acute COVID-19 and in long COVID.
2. Fibrin's Interaction with the Virus: The virus responsible for COVID-19, SARS-CoV-2, binds to fibrin, making the clots it forms more harmful. These clots can be more difficult to break down, leading to persistent inflammation and contributing to long-term symptoms, especially those related to the brain, like brain fog.
3. Impact on the Brain and Immune System: Fibrin deposits in the brain can lead to inflammation and damage to nerve cells. It can also interfere with the immune system, particularly natural killer (NK) cells, which are crucial for fighting off infections. By suppressing NK cells, fibrin helps the virus evade the immune response, making it harder for the body to clear the infection.
4. Potential Treatments: The study suggests that targeting fibrin with specific antibodies could be a way to reduce these harmful effects. These treatments might protect against the inflammation and nerve damage caused by COVID-19, potentially offering a therapeutic option for both acute and long COVID.
Summary of Treatment Potential
1. Targeting Fibrin with Antibodies: The study suggests that a specific type of monoclonal antibody (referred to as 5B8) that targets the inflammatory domain of fibrin could help reduce the harmful effects of fibrin in COVID-19. This antibody has been shown to protect against both lung and brain inflammation and damage.
2. Reducing Inflammation: The antibody treatment reduces the activation of immune cells like macrophages and microglia, which are involved in the inflammatory response in the lungs and brain. This could prevent or lessen the severity of symptoms like lung fibrosis and neuroinflammation.
3. Improving Immune Response: The treatment with the fibrin-targeting antibody also appears to enhance the function of natural killer (NK) cells, which are important for controlling viral infections. By preventing fibrin from suppressing these cells, the treatment might help the body clear the virus more effectively.
4. Preventing Neurological Damage: The antibody treatment may protect against the neurological damage seen in COVID-19, such as brain fog and other cognitive issues. It does this by reducing fibrin deposits in the brain and limiting the inflammatory response that can harm neurons.
5. Potential for Long COVID: Given that fibrin-related inflammation and clotting issues can persist in long COVID, this treatment could also be beneficial for patients suffering from long-term symptoms after their initial infection.
6. Broad Applicability: The treatment has shown effectiveness in different models and against various strains of the virus, suggesting it could be broadly applicable regardless of the specific form of COVID-19 or whether the virus directly invades the brain.
How targeting fibrin could become a new therapeutic strategy for managing both the acute and long-term effects of COVID-19.
More on 5b8
5B8 is a monoclonal antibody specifically designed to target a particular region of the fibrin protein, which is involved in blood clotting and inflammation. In the context of COVID-19, fibrin has been found to play a significant role in driving inflammation and tissue damage, particularly in the lungs and brain.
Key aspects of 5B8:
- Targeting the Inflammatory Domain: 5B8 binds to the inflammatory domain of the fibrin protein, specifically the γ377–395 region. This region of fibrin is known to trigger immune responses that can lead to inflammation and tissue damage.
- Preventing Harmful Effects: By binding to this region, 5B8 prevents fibrin from interacting with other immune cells that would normally cause inflammation. This helps reduce the overall inflammatory response in the body.
- Therapeutic Potential: In studies, the use of 5B8 has been shown to protect against lung and brain inflammation, reduce the severity of lung fibrosis (scarring), and protect neurons (nerve cells) from damage. This makes it a promising candidate for treating both the acute and long-term effects of COVID-19, including conditions like long COVID.
- Broad Application: 5B8 has shown effectiveness in different experimental models of COVID-19, suggesting it could be useful across various stages of the disease and different viral strains.
Who Currently Makes 5B8?
The monoclonal antibody 5B8 is developed by Beth Israel Deaconess Medical Center, Inc. This antibody targets a specific epitope on fibrin and is part of a broader research initiative to create therapies that address neuroinflammation without affecting coagulation.
If 5B8 works, how long would it take to manufacture?
The production of monoclonal antibodies like 5B8 involves a complex and time-consuming process. Here is an overview of the timeline and considerations for producing monoclonal antibodies at a scale sufficient for widespread distribution:
Production Timeline
- Initial Development: Creating a new monoclonal antibody from scratch can take approximately 12 months. This includes the initial steps of designing the antibody, developing the production cell line, and optimizing the production process.
- Manufacturing Process: Once a cell line is established, the actual manufacturing process for an existing antibody takes about 3-4 months. This involves thawing a vial from an existing cell bank, culturing the cells in bioreactors, and purifying the antibodies.
- Total Time to Market: Including regulatory approvals and further development phases, the entire process from initial development to having a product ready for distribution can take around 18 months.
Production Capacity
- Scale of Production: Large-scale production of monoclonal antibodies involves using mammalian cell culture systems, which can produce significant quantities of antibodies. However, the scale of production is limited by the capacity of bioreactors and the complexity of the manufacturing process.
- Feasibility for Whole Population: Producing enough monoclonal antibodies to supply the entire population is challenging. The process requires substantial infrastructure, resources, and time. While advances in technology have improved production efficiency, scaling up to meet global demand remains a significant challenge.
It is possible to produce monoclonal antibodies like 5B8 on a large scale, doing so for an entire population would require considerable time, resources, and coordination. The complexity and cost of production, along with the need for specialized facilities, pose challenges to achieving this level of production, but it is possible.